In the global effort to combat climate change and reduce dependency on fossil fuels, industries worldwide are being called upon to make fundamental changes. For the pharmaceutical sector, this means shifting towards sustainable practices in logistics, packaging, and overall supply chain management.
Traditionally, pharmaceutical manufacturing has been highly competitive with a heavy focus on regulatory compliance and patient safety. However, as the urgency of climate change grows, the sector must adapt, harmonizing sustainability with other business goals. Contract Development and Manufacturing Organizations (CDMOs) play a vital role in pharmaceutical supply chains and are uniquely positioned to drive this transformation.
Collaboration with clients, suppliers, sustainability consultants, tech providers, and packaging firms can help pharmaceutical companies drive progress towards their sustainability goals. CDMOs stand at the center of this ecosystem, influencing decisions that can reduce environmental impact and encourage best practices across the entire supply chain. By promoting sustainable logistics practices and fostering strong partnerships, CDMOs can help shape a greener future for the pharmaceutical industry.
Shared Responsibility: Aligning with Client Goals and Industry Trends
Today’s leading pharmaceutical companies have taken drastic steps to reduce carbon emissions, minimize waste, and conserve resources like water and energy. According to a 2023 study by the World Economic Forum, the pharmaceutical industry accounts for approximately 4.4% of global carbon emissions, with a majority stemming from supply chain activities1. For large companies, this push toward sustainability is often part of a broader corporate strategy. However, for smaller firms or start-ups that may not have the same resources, sustainability can sometimes take a backseat. This is where CDMOs can add value—not only meeting client needs but also guiding them and suppliers toward more environmentally responsible practices. By actively offering advice and support, CDMOs can help smaller clients and suppliers achieve their goals in ways that are both sustainable and economically feasible.
Simplifying Complex Supply Chains to Reduce Emissions
Pharmaceutical supply chains are notoriously complex, and over 70% of emissions in the life sciences sector originate from these activities1. Much of this impact is tied to logistics, as companies transport materials, ingredients, and finished products across regions. In addition to these logistical demands, the pharmaceutical industry faces stringent regulations around product quality, safety, and transportation conditions. For instance, many pharmaceutical products require temperature-controlled environments, which add layers of complexity and contribute to emissions.
Rachel Griffiths, Director of Technical Services at PCI Pharma Services, emphasizes that the logistics challenges CDMOs face often stem from the global nature of clinical trials and production. “Products may be manufactured in one location then distributed to clinical trial sites worldwide, where we often have limited control over the specific sites. In these situations, CDMO’s must collaborate closely with logistics partners to assess every available option, seeking ways to reduce travel distances and optimize routes.”
One approach Griffiths highlights is the focus on minimizing “air miles”—the number of miles products travel by air, which has a significant carbon impact. For instance, if a product manufactured in Europe is requested for packaging in the U.S. and then returned to Europe for distribution, it’s important to explore alternatives to avoid such back-and-forth trips. “We take this into consideration during the site selection process within the PCI network as well, recommending local sites to our clients where appropriate to minimize environmental impact,” adds Griffiths. Establishing local supply sources, where possible, allows high standards to be maintained while minimizing the environmental footprint of these shipments.
Moving from Single-Use to Reusable Shipping Containers
Temperature-controlled shipping solutions—often essential for pharmaceutical logistics—traditionally involve single-use containers. These containers generally require disassembly, separating out different components for recycling. This process is not only labor-intensive but also generates considerable waste and consumes energy, making it an inefficient and environmentally costly solution. CDMOs can address these challenges by transitioning to reusable shippers.

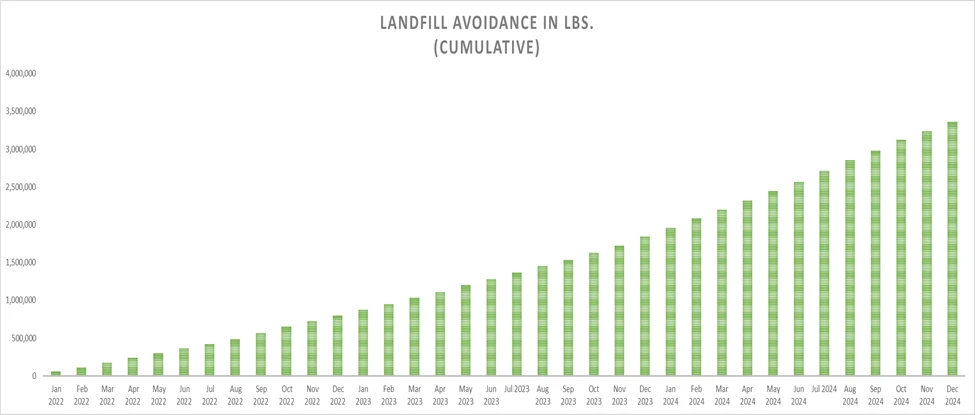
PCI’s collaboration with Cold Chain Technologies, for example, has led to significant reductions in waste. According to a life cycle assessment provided by Cold Chain Technologies, switching to reusable shippers has prevented nearly 2 million pounds of waste from ending up in landfills over a two-year period by using reusable shippers. The environmental benefits of this switch are clear. For every 100 EcoFlex shippers deployed instead of comparable single-use containers, PCI saves an estimated 16 trees, 434 liters of oil, 390,423 liters of water, and nearly 1,000 pounds of waste. This move is not only an important step in landfill reduction but also demonstrates the measurable impact of adopting sustainable logistics practices.
Packaging Innovations to Optimize Sustainability
Beyond reusable shipping solutions, there are other ways to reduce the environmental impact of pharmaceutical logistics. One such method is rethinking packaging design. More efficient, recyclable packaging solutions reduce the need for materials and minimize empty space in shipping containers, which lowers overall shipping volume. By adopting these streamlined packaging approaches, CDMOs can enhance sustainability while also optimizing cost efficiencies.
Cold Chain Logistics: Facing the Sustainability Challenge
The rise of cell and gene therapies has added a new level of complexity to pharmaceutical logistics. These therapies require ultra-cold chain logistics, with temperatures that can dip below -25°C. The manufacturing processes for these treatments are intricate, making it difficult to transfer production to other locations, which increases the need for innovation in sustainable cold-chain logistics.
Looking ahead, the industry is starting to see significant advancements in renewable energy sources for transportation and refrigeration. Innovations in this area are crucial to reducing the pharmaceutical industry’s carbon footprint. Dry ice, currently indispensable for maintaining sub-zero temperatures, poses another sustainability challenge. Although effective, it is derived from CO2, and finding more environmentally friendly alternatives will be key for the industry as it strives to reduce its reliance on fossil fuels.
Embracing ESG Principles as a Core Business Strategy
The drive for sustainability in the pharmaceutical sector is not solely due to pressure from clients. CDMOs recognize that they have a responsibility to lead by example and promote sustainable practices within the complex network of suppliers and stakeholders involved in delivering medications to patients. Moving beyond rhetoric, companies in the life sciences sector must show concrete progress toward sustainability goals.
CDMOs are increasingly partnering with organizations like EcoVadis to establish sustainability baselines and benchmarks, which serve as the foundation for improvement. Working with the Science-Based Targets Initiative, CDMOs are setting ambitious goals aligned with the Paris Agreement, demonstrating a commitment to tracking both short- and long-term progress.
PCI Pharma Services has made significant strides in this area, implementing numerous impactful initiatives. These include rolling out reusable shippers, launching a sustainable procurement program, adopting recyclable packaging, and increasing the use of renewable energy. PCI is also finalizing a comprehensive ESG strategy, with the goal of achieving net-zero emissions by 2045. This strategy is built on insights gathered from surveys of investors, customers, employees, and local communities, which have helped PCI identify nine key impact areas: carbon footprint, energy efficiency, waste management, water conservation, labor and human rights, health and safety, diversity, equity and inclusion, community impact, and sustainable procurement.
PCI’s ESG Report outlines these initiatives and provides clear targets for future progress. The company has also established an ESG-focused steering committee with cross-regional representatives, ensuring that sustainability efforts are embedded across all levels of the organization. This comprehensive approach reflects PCI’s commitment to transparency and its willingness to lead the way toward a more sustainable industry.
Shaping the Future of CDMOs: Meeting the Demands of a Sustainable Supply Chain
The pharmaceutical industry’s largest players are increasingly prioritizing Scope 3 net-zero targets, which include emissions from their supply chains. As a result, they are placing greater emphasis on the sustainability credentials of their suppliers, including CDMOs. For CDMOs to meet these expectations, collaboration and innovation will be essential. They must work closely with suppliers to standardize data collection, improve reporting accuracy, and leverage new technologies that support sustainability.
One emerging trend is the adoption of power purchase agreements, where CDMOs and their partners commit to purchasing renewable energy. Additionally, by sharing best practices and innovations, CDMOs and their suppliers can collectively drive the industry toward more sustainable solutions. The role of CDMOs in this transformation cannot be overstated; as gatekeepers of the supply chain, they are uniquely positioned to lead the pharmaceutical industry’s journey toward sustainability.
Looking Ahead: Building a Sustainable Future Together
CDMOs like PCI Pharma Services play a critical role in creating a more sustainable pharmaceutical supply chain. Through a combination of strategic partnerships, innovative logistics practices, and a commitment to ESG principles, CDMOs can help their clients reduce their environmental impact and meet their sustainability targets. Recently, PCI put this into practice by participating in Marken’s Green Investment Pilot, which helped them achieve an absolute emissions reduction of over 18% across participating accounts. The shift to sustainable logistics and supply chain practices is not a simple process, but with collaboration and innovation, the pharmaceutical industry can achieve meaningful change.
As PCI continues to invest in sustainable practices, the company sets a powerful example for the entire industry. By working together with clients, suppliers, and other stakeholders, CDMOs can make a lasting impact on the environment, fostering a more sustainable and resilient future for healthcare.
Sources:
- Sumudu Dehipawala, Ellie Goldman, Emily Hwang, Prem Shah, Ayushi Shroff, and Matthew O’Hara, The pharmaceutical industry’s carbon footprint and current mitigation strategies: A literature review, Trinity Life Sciences, accessed August 16, 2023.
We are committed to supporting clients at every stage of the manufacturing cycle, delivering best-in-class services efficiently and effectively.
Find out more about our fully integrated Packaging Services.





