With the number of registered clinical trials increasing significantly each year, it’s not surprising to learn that the clinical trials supply and logistics market is predicted to grow exponentially in the years ahead. Recent data suggests that by 2030 this market will be valued at approximately $12.4 billion, up from $5.1 billion in 2020; this translates to a Compound Annual Growth Rate (CAGR) of 9.1% from 2021 to 2030.1 Such growth places huge pressure on drug discovery companies, CROs and CDMOs to find supply management solutions which ensure seamless, efficient delivery of clinical trial materials worldwide, whilst navigating the challenges of more complex study designs and the ever-evolving regulatory landscape. PCI Pharma Services’ clinicalSMART™ is one such solution.
What is clinicalSMART™?
PCI’s clinical Supply Management And Readiness Team (SMART) is a department of experienced Clinical Supplies professionals who manage clinical drug supply on behalf of clients. Currently, PCI’s clinicalSMART™ is supporting 74 clients and 214 studies globally. Designed with flexibility at its core, clinicalSMART™ can support sponsor requirements either throughout the entire study lifecycle, from protocol development through to the final destruction of materials, or at time points when sponsor teams require additional resources to supplement in-house supply management expertise.
It does so by collating all key information from external parties (such as trial design, drug stability, and recruitment assumptions) and utilizing in-house expertise to develop optimal supply strategies. Whether working with clients’ internal teams in a consultative role or assuming full ownership of supply chain management, clinicalSMART™ services are immediately available, preventing the need to recruit new staff, and delivering the agreed contracted hours per month as required by the client.
Established in 2016, clinicalSMART™ addressed the industry’s growing need for integrated clinical supply management services. At that time, the two highest-ranking therapeutic areas in terms of clinical trial cost per patient were hematology and oncology, with a median cost of over $200k and over $100k respectively.2
As of September 2022, there were 174,669 registered interventional clinical trials using drug or biologic therapies.3 Considering the sheer numbers involved here, any delays, miscommunications or errors in the clinical trials management process would be extremely costly in financial terms, not to mention the most important factor: the risks to the patients themselves.
Due to its initial successes and a growing need from clients outside the US for the services offered by clinicalSMART™, the European and Asia Pacific team was established in 2019 to create a global team.
Experience, Expertise and Services
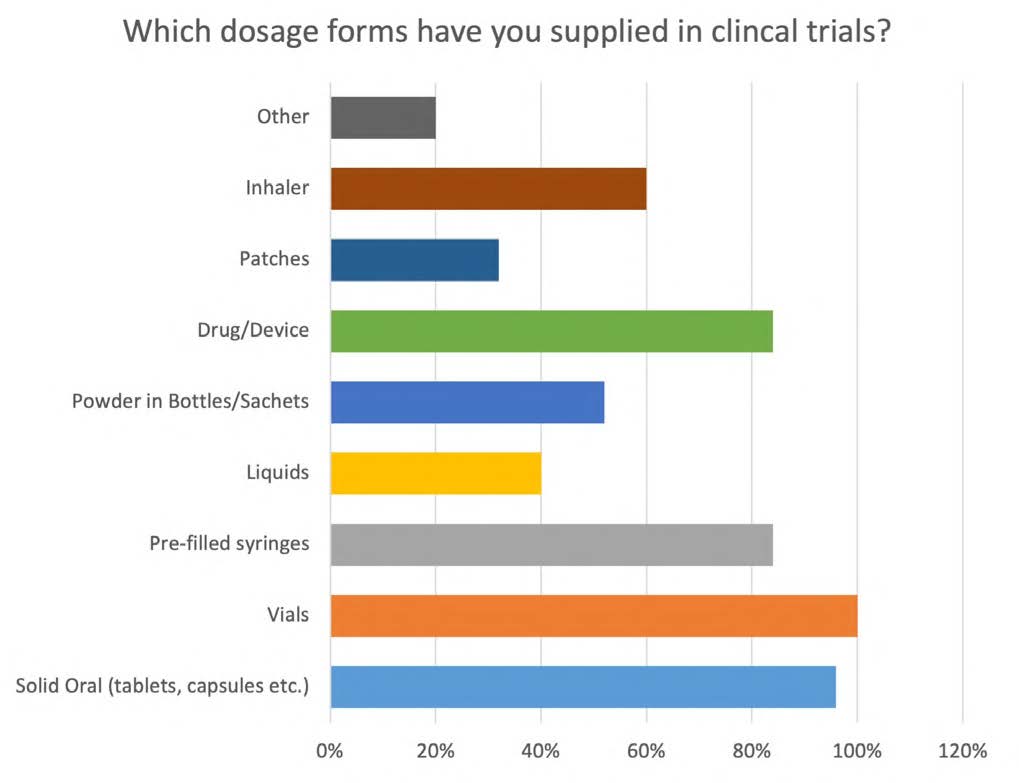
PCI’s clinicalSMART™ team has a collective experience of almost 400 years, with an average of 16 years’ clinical trials supply experience per Clinical Supply Manager (CSM). In a recent survey, CSMs noted their level of experience and expertise in terms of therapeutic areas supported, previous employment, dosage forms supported, and a list of the services provided in their tenure with PCI’s clinicalSMART™, as outlined in Figures 1 to 4 below.
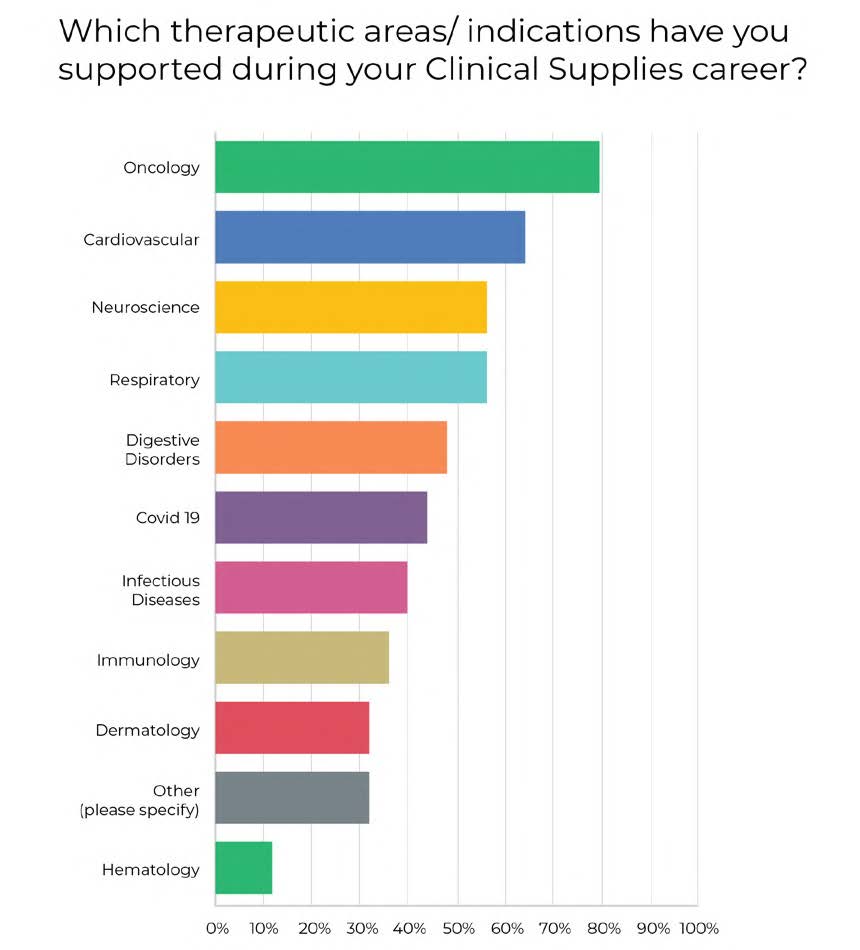
Figure 1: Category Other includes reproductive, pain management, liver disease, hypertension, autosomal recessive disorder, gastrointestinal, rare diseases, cell and gene therapy, generic products, central nervous system.

Figure 2
RFQ Support Services
In addition to clinical supply chain management, clinicalSMART™ is also able to initiate RFQ document writing to support outsourcing requirements on a client’s behalf. After reviewing key study assumptions and estimates in order to establish an initial study plan, the RFQ and relevant supporting documentation is prepared for the client; due to a thorough knowledge and evaluation of the study requirements, a high level of accuracy within the quotation is assured. As with the study plans, RFQs are then submitted to the clinicalSMART™ team for peer review, drawing on the vast experience within the team to ensure a high level of accuracy.
How does clinicalSMART™ operate?
The effectiveness of clinicalSMART™ is best described via two real-world case studies.
Case Study #1: Integration and Embedded Support
In one instance, PCI was performing packaging and global distribution for a client with a very aggressive clinical plan involving 7 global trials, complex multi-dose carded kits, and many inventory items. At a crucial stage, the client’s CSM resigned, creating a gap in support. In response, a clinicalSMART™ CSM was contracted to provide supply study oversight.
The clinicalSMART™ CSM’s role included:
- Responsibility for planning and creating demand and supply schedules;
- Working with the Project Manager to allocate inventory and stabilise the packaging schedule per trial
- Working with the Distribution Project Manager to establish planned depot shipments;
- Eliminating rushed packaging and shipments.
Not only did the clinicalSMART™ CSM’s involvement maintain the drug supply for all trials without slowing the enrolment process, but they also worked with the client to recruit their own CSMs and trained them to assume all responsibilities going forward.
This seamless integration into the client’s supply chain is key to the clinicalSMART™ philosophy, as is the multifaceted nature of the clinicalSMART™ CSM’s role. They serve as a Project Manager.
| Answer Choices | Responses |
|---|---|
| Forecasting for IMP (initial or re-supplies) | 85.71% 18 |
| Acting as unblinded Supplies Team Member for blinded clients | 80.95% 17 |
| Inventory management for IMP, IMPs or ancillaries either manually or via IRT | 80.95% 17 |
| Distribution strategies (for initial or re-supplies) | 76.19% 16 |
| IRT study maintenance (e.g. lot release, expiry updtes, kits status updates and trigger resupply updates) | 76.19% 16 |
| Drug supply strategies (for initial or re-supplies) | 71.43% 15 |
| Expiry date management (depot and site level) | 71.43% 15 |
| Acting as Client Supplies Team Member (i.e.: no client resource) | 66.67% 14 |
| IRT user acceptance Testin (UAT) | 66.67% 14 |
| Drug accountability, returns and reconciliation | 66.67% 14 |
| GMP document review | 61.90% 13 |
| Project timelines- Initial supply or resupply | 57.14% 12 |
| Label design (English Text) | 57.14% 12 |
| IRT system development (Specifications, UAT) | 57.14% 12 |
| Creation of study documents (e.g. pharmacy manuals etc.) | 57.14% 12 |
| RFP development | 57.14% 12 |
| Protocol review | 52.38% 11 |
| SOP or process development | 52.38% 11 |
| Kit design | 47.62% 10 |
| Labelling strategies | 47.62% 10 |
| Forecasting for IMPs or ancillaries (initial or re-supplies) | 42.86% 9 |
| Risk management- Study maintenance | 33.33% 7 |
| Budget reconciliation | 33.33% 7 |
| Risk assessment- Stud start | 28.57% 6 |
| Other (please specify) | 28.57% 6 |
| Total Respondents: 21 |
Figure 4: Category Other includes Onboarding and interviewing new sponsor staff, supporting client CSMs, FIFO Assessment, Import/Export including Duty/Tax implications, supporting
client vendor selection.
Aiding communication between PCI, CMC, the Clinical team, Regulatory, CRO partners and IRT vendors; a client advocate who understands the needs of both the client and PCI and will bridge the gap between the two parties, ensuring that nothing is lost in translation; a continuous liaison throughout the trial lifecycle; a team member able to provide a Full-Time Equivalent (FTE) without the overheads involved in adding headcount; and an unblinded resource, which is critical to managing a controlled supply chain. These services are underpinned by a high level of reassurance that the clinical supply management is in the hands of a highly experienced team, with almost 400 years collective experience in this field of expertise.
Case Study #2: Plan, Execute, Review – APost-Go Live Intervention
Limited staff resources within client organisations is another potential cause of delays, threatening the timing of trial execution. One client contracted a SMART™ CSM to manage unblinded IRT activities and drug inventory at sites, with the contract established as the trial started when the client realised they had no unblinded personnel.
This began as an IRT support related role for the CSM, with the client responsible for all planning and packaging oversight, and the PCI CSM’s role commencing after sites had been seeded with initial supplies. Almost immediately, the PCI CSM determined that the quantity of drug packaged and supplied in initial site shipments was insufficient to support ongoing site re-supplies until the end of treatment period. The PCI CSM alerted the client to this critical risk only weeks into the trial lifecycle.
The SMART™ CSM immediately started working with the Project Manager and Distribution PM to establish a solution for the client, which involved:
- Creating a new supply plan to fit the trial needs;Preparing a rushed packaging operation to generate more drug supply;
- Performing a number of site-to-depot returns and site-to-site drug transfers;
- Maintaining manual monitoring of drug inventory and site shipments.
Following the implementation and execution of the new plan, trial activities were maintained without interruption. Sites continued to open on schedule, enrolment continued without any slowing down, and the trial design was expanded to increase the number of patients. The final delivery of this clinical trial led to this client contracting a portfolio of trials to the PCI clinicalSMART™ team.
This case study is an illustration of clinicalSMART™ performing their Plan, Execute, Review (PER) model, which is a core process that can be applied throughout the study lifecycle. It ensures a balance of the actuals of a clinical trial against the initial assumptions and real-time reactions to any potential risks to the study.
This experience-lead planning involves a deep understanding of stakeholder needs, and the ability to challenge vendors to ensure all aspects of the supply chain are being considered. These plans are then peer-reviewed, a process of internal critique which ensures that plans are robust and any risks are identified, assessed, and managed appropriately. During execution, clients benefit from full-time supply chain support and back-up support where necessary, with optimal issue avoidance resulting from the support of almost 400 years experience across the team. Plans are then reviewed after execution to assess lessons learned, and to share these lessons within the team. Overall, this creates a proactive supply chain management process, instead of a reactive one where issues are not identified in good time.
When to get SMART
Regardless of the level of CSM involvement contracted to the client, the critical objective remains the same: to provide a seamless, integrated clinical supply service at the level required by the client, at any stage or throughout the study lifecycle, whilst leveraging PCI’s extensive experience in this area of expertise.
However, to gain maximum benefit from clinicalSMART™ it is recommended that services are contracted as early as possible, preferably prior to study start up. This enables the PCI CSM to collate and analyse key information from external study stake holders, and to review the protocol, the drug stability, the availability of comparators in the marketplace, the countries involved in the study, the labeling and distribution strategy, and recruitment projections.
This comprehensive assessment will help the clinicalSMART™ CSM identify the least wasteful kit design and create an initial packaging forecast, which can be modified as changes arise. The CSM will also ensure the forecast is ‘fit for purpose’ by assessing it against the potential drug utilisation in the IRT.
The PCI CMS will typically review the IRT specifications and recommend initial shipment triggers and re-supply values, ensuring best use of available drug product, adjusting values when live study data becomes available, and when changes arise from unplanned protocol revisions, or variance between projected vs actual site activity. This enables the PCI CSM to flag potential supplies risks, and present corrective measures.
The Future of SMART
Since its conception, clinicalSMART™ has grown exponentially with the number of trials supported globally increasing year on year. PCI is committed to an ongoing recruitment plan to help facilitate this growth in the years to come. PCI is proud to have clinicalSMART™ as part of its service offering, and its continued excellence in providing seamless, integrated services aligns perfectly with the wider organisation’s goal of providing true end-to-end CDMO services for clients around the globe. If you want to learn more about clinicalSMART™ and how it may assist you, please get in touch today.
1. https://www.biospace.com/article/clinical-trial-supply-and-logisticsmarket-size-share-growth-trends-forecast-2021-to-2030/?keywords= COVID+19+vaccine
2. https://www.statista.com/statistics/1197095/clinical-trial-cost-per-patientby-therapy-area/ 3. https://www.clinicaltrials.gov/ct2/resources/trends#TypesOf RegisteredStudies
Carolyn Timpany Senior Supplies Manager for the International Region at PCI Pharma Services, joined PCI in July 2022 from PPD, where she held the positions of Client Oversight Managerand SeniorSupplies Manager. She has a breadth of experience in Clinical Supplies Management spanning a period of 17 years, and was formerly a Project Manager, Business Development Manager and Integrated Process Manager IRT/ Supplies at theAlmac Group.
Lisa Spence Director, Clinical Supply Chain for the International Region at PCI Pharma Services, joined PCI in March 2019 and has over 25 years clinical supply experience within in the pharmaceutical industry which was gained whilst working at GSK, Merck Sharp & Dohme, Pfizer and MedImmune. Lisa has industry expertise in clinical supply manufacturing, packaging, labeling/distribution, clinical supply chain project/programme management, regulatory and clinical operations. Lisa also has a BSc. Hons. degree in Pharmaceutical Science, which she gained at Greenwich University in 1995. Since joining PCI Lisa has created a newteam of clinical supplymanagers that provide clinical supply management support for PCI’s EU/ ROW customers.
Ed Groleau Director, Clinical SupplyChain for North America at PCI Pharma Services, has over 30 years of experience in the Pharmaceutical industry. He joined PCI Pharma Services in 2018 and became the director of his group in 2019. Ed leads the Supply Management And Readiness Team (SMART) at PCI where his team partners with clinical trial sponsors to provide any clinical supply management services from protocol development through destruction. Prior to PCI, Ed worked in numerous departments at Eli Lilly, spending 15 years in various laboratories before moving into the Clinical Trial Supplies group in 2003. In 2011 he became part of a highly integrated CM&C team responsible for overseeing the development of compounds from discovery through the proof-of-concept stage. In 2016 Ed moved to Elanco, Lilly’s animal health division, where he established a global clinical trial supplies group for developing companion and food animal projects.
We are committed to supporting clients at every stage of the manufacturing cycle, delivering best-in-class services efficiently and effectively.
Find out more about our fully integrated SMART™ Services.




