We provide complete pharmaceutical development and manufacturing services for both investigational and commercial products including highly potent molecules requiring specialist handling. Complemented by specialist high potent packaging suites, we are able to take the most potent of products from development to launch.
High Potent Oral Dose
Our comprehensive pharmaceutical development service offering includes new drug development, early stage formulation, and analytical development for both highly potent and non-potent drug products.
Our strength lies in the integrated nature of our services, combining formulation and analytical development with GMP clinical manufacturing and packaging through a full cross functional project team, coordinated by an experienced team of project managers.
Utilizing our state-of-the-art facilities, we offer unrivaled capabilities and a true focus on customer needs. We provide clinical manufacturing of multiple dosage forms for investigational use including solid oral dose, liquids, and semi-solids.
We are able to offer API directly into capsules and vials using microdosing technology as well as the more traditional development pathways. The microdosing drug in capsule (DIC) and drug in vial (DIV) approach offers time and cost efficiencies as well as the potential to reduce wastage of often expensive APIs.
Following early stage development, we continue with further development, scale-up, and process validation ahead of commercial launch for a variety of dosage forms, all supported by full in-house analytical development and release services.
All clinical manufacturing services are fully supported by a dedicated and experienced team of Qualified Persons, a full analytical laboratory, and a GMP-compliant warehouse.
High Potent Sterile Fill-Finish and ADCs
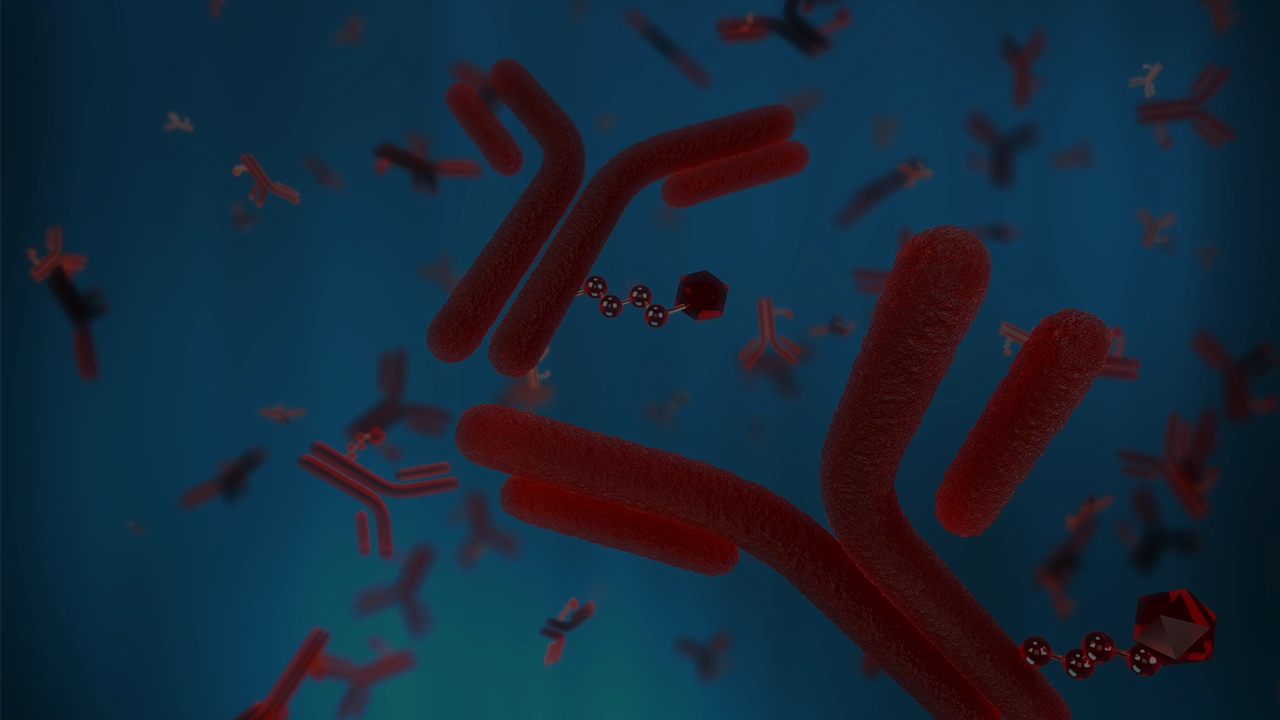
Antibody-drug conjugates (ADCs) are transforming oncology treatment and at PCI, we believe that selecting the right CDMO for your new ADC is as important as selecting the right ADC components. As a partner, we offer stability, quality, strategic alignment and a forward-thinking approach in the development and manufacturing of your ADC product, supported by full, temperature-controlled packaging, storage and distribution services from our clinical packaging sites globally.
The components required for the successful development of ADCs include Sterile fill-finish, lyophilization and specialist high-potency processing capabilities.
At PCI, our Integrated ADC Development Services include:
- Development, optimization and manufacturing services
- Process development, process optimization and scale up
- Formulation development (liquid and lyophilized) and drug product development
- Regulatory support
In addition, our experienced analytical teams will ensure a successful development program. As a global leader of sterile fill-finish, lyophilization and high potent manufacturing services, a dedicated team will support your product lifecycle with method development, method optimization, method transfer and method qualification/validation. To complete our end-to-end service offering and driven by innovation and patient-centricity, our packaging design and development expertise, drug device final assembly, pack and test, and advanced drug delivery packaging capabilities offer flexible solutions for a diverse portfolio of conventional and specialty injectable drug products.